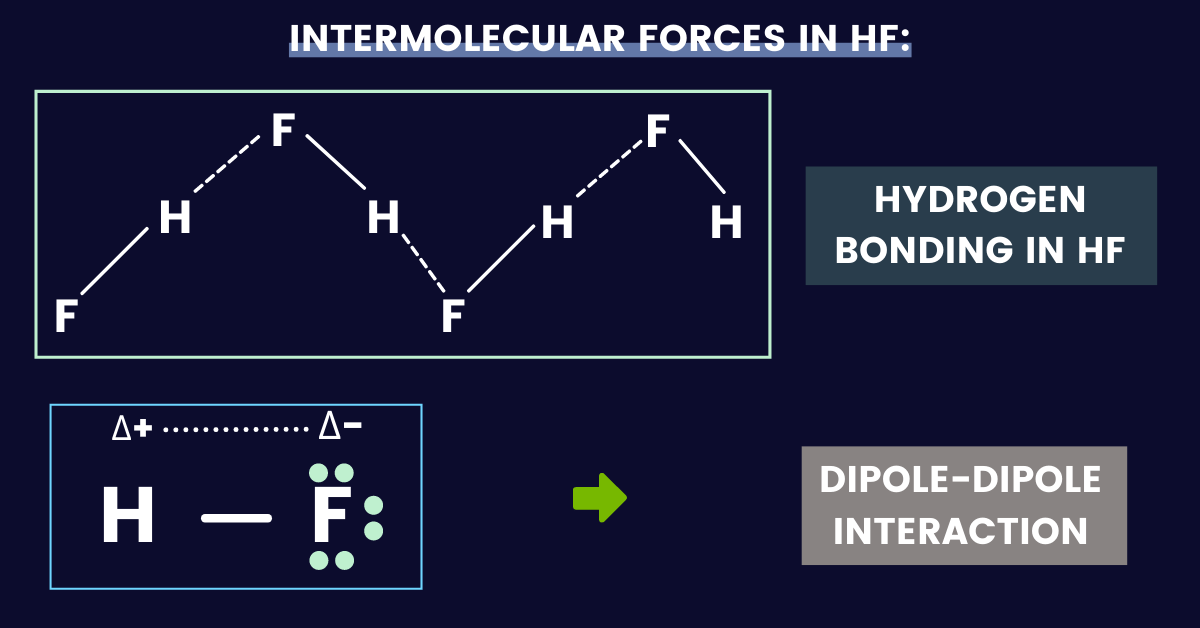
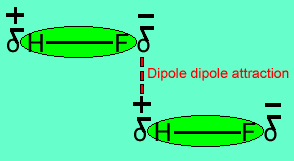
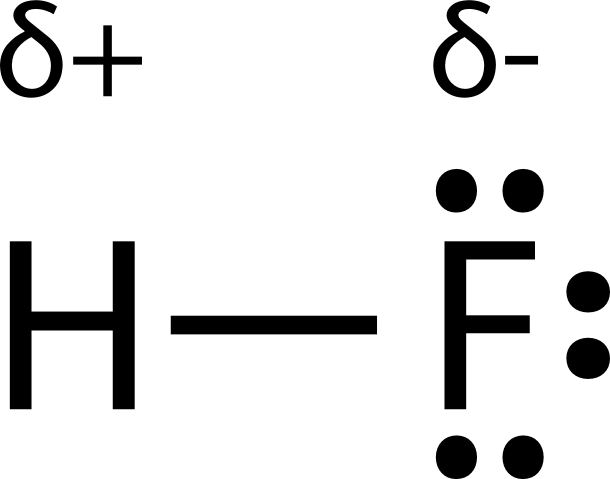Name two intermolecular forces that exist between HF molecules in liquid state. | CLASS 11 | STA... - YouTube

SOLVED: In the liquid state; which species has the strongest intermolecular forces, CH4, Ch, Oz or HF? CHA Clz 0z D. HF Which of the following has London dispersion forces as its

Intermolecular attractions. Thus far… Bonding covered so far involved intramolecular bonding, i.e. bonding within a molecule or within an ionic crystal. - ppt download

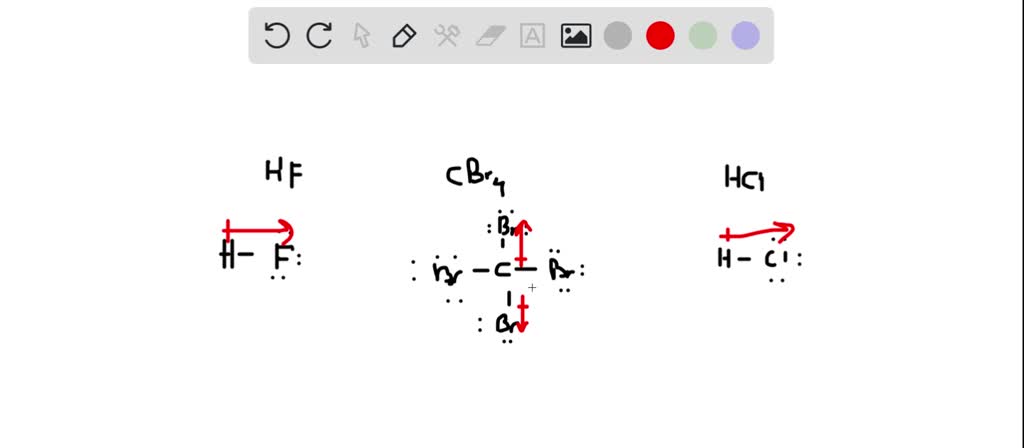
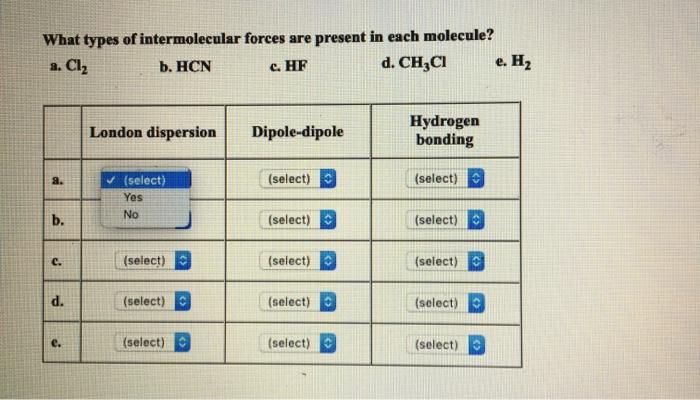
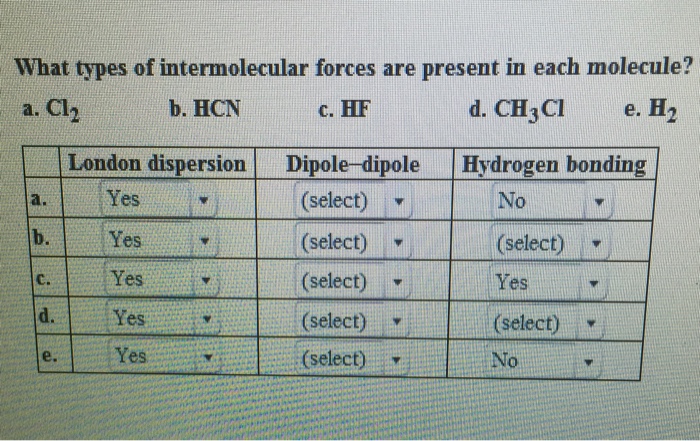
SOLVED: QUESTION 3 Identify the important intermolecular forces in HF dipole-dipole dispersion ion-dipole hydrogen bonding
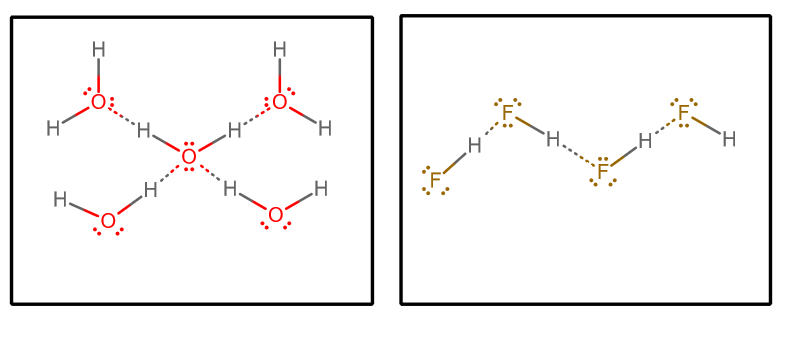
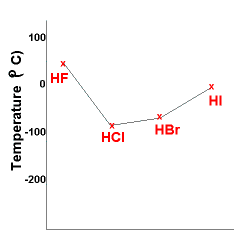
Why does "HF" have a lower boiling point than water even though "F" is more electronegative than "O"? | Socratic

name intermolecular forces that exist between HF molecules in liquid satate - Chemistry - States of Matter - 12014515 | Meritnation.com

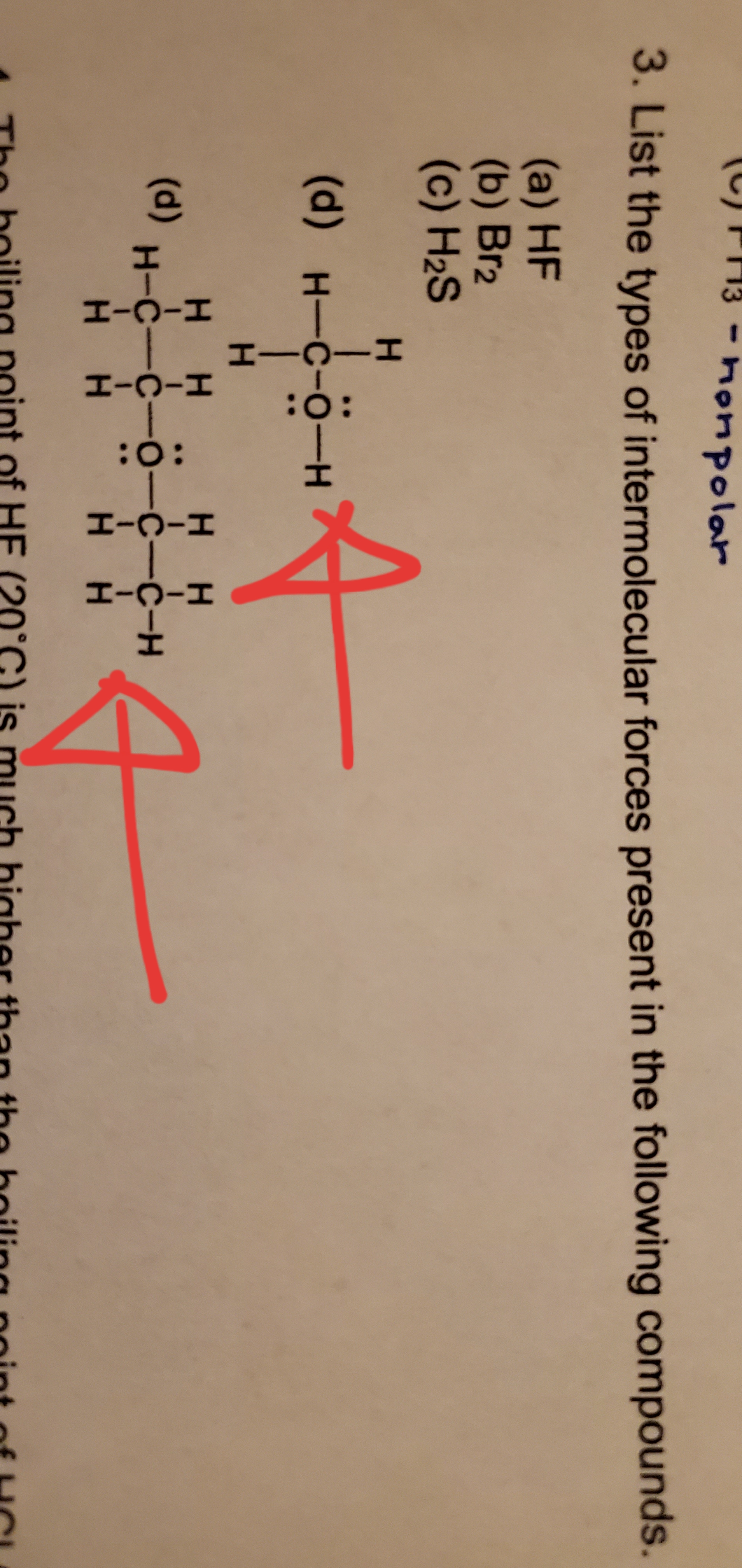
Identify the dominant type of intermolecular force present in the following: a) \ HF\\ b) \ CH_2=O\\ c) \ NaCl\\ d) \ O_2 | Homework.Study.com